And It's About Time There Was Some Support For Cushing's!
Marie Simard, M.D.
Neurosurg Focus 16(4), 2004. © 2004 American Association of Neurological Surgeons
Posted 05/13/2004
Abstract and Introduction
Abstract
Cushing syndrome is an insidious illness that warrants an early diagnosis to avoid the effects of prolonged hypercortisolism. The variability in the clinical features of the disease and the occasional inconsistencies between different biochemical tests performed to identify it render the diagnosis challenging. In this paper the author discusses the various biochemical tests that are useful for the diagnoses of Cushing syndrome and Cushing disease, with an emphasis on the respective sensitivities and specificities of these tests. The measurement of evening salivary cortisol and the combined low-dose dexamethasone–corticotropin-releasing hormone stimulation test have improved overall sensitivity and specificity in the evaluation of Cushing syndrome and Cushing disease. Introduction
A diagnosis of Cushing syndrome, unless unequivocal, is often a challenge for the clinician. The signs and symptoms of Cushing syndrome are often subtle during the initial stage of the illness. Cushing syndrome may present as an episodic hypercortisolism that is associated with fluctuating clinical findings; however, due to the detrimental effects of prolonged hypercortisolism on body tissues, early detection of the disorder is essential.[20] In this paper I will review the various screening tests used to determine hypercortisolism (Cushing syndrome) and the biochemical evaluation performed to identify a pituitary ACTH-secreting adenoma (Cushing disease).
Cushing syndrome has an incidence estimated to be approximately 10 per 1 million persons.[23] The ACTH-dependent forms of this syndrome include the following: 1) pituitary corticotroph adenomas or Cushing disease;[40] 2) syndrome of ectopic ACTH secretion by tumors originating in the lung, bronchus, thymus, or pancreas pheochromocytomas, medullary thyroid carcinomas, and ovarian steroid-cell tumors; and 3) a rare ectopic CRH secretion that usually originates from pheochromocytomas, gangliocytomas, and paragangliomas.[2,34,38] Cushing disease accounts for between 70 and 80% of ACTH-dependent forms of hypercortisolism[24] and for 15% of all pituitary adenomas in adults.[40] The ACTH-secreting tumors are four- to sixfold more prevalent in women[27] and are found in patients who are predominantly between the ages of 20 and 60 years.[40] The ACTH-secreting pituitary tumors represent approximately 55% of pituitary adenomas that are diagnosed in children 11 years or younger and approximately 33% of such tumors diagnosed in patients younger than 20 years of age.[18] The disease is equally common in prepubertal boys and girls.[16] Cushing disease is most often caused by a solitary intrasellar microadenoma. Pituitary microadenomas are identifiable in more than 90% of adults and in 80 to 85% of children and adolescent patients with Cushing disease.[25] Those patients who do not have an identifiable adenoma may experience a primary hypothalamic dysfunction.[25] Macroadenomas account for up to 10% of corticotropinomas, with invasiveness exhibited more frequently among younger patients.[27] Nodular corticotroph hyperplasia without evidence of a CRH-secreting neoplasm has been reported in 2% of surgical cases or less.[42] A subset of older patients with nonsuppressable, long-standing ACTH-secreting adenomas may present with a macronodular adrenal disease by autonomously secreting cortisol.[22]
Patients with Cushing syndrome may present with various signs and symptoms (Table 1). Clinical features more suggestive of Cushing syndrome include facial plethora, increased supraclavicular fullness, central obesity, proximal muscle weakness, cutaneous wasting (thickness of skin on the dorsum of the hand < 2 mm), purple striae wider than 1 cm, spontaneous ecchymosis, osteopenia, hypertension, and, in children, early or delayed puberty and growth retardation with delayed or advanced bone age.[16,20,28] Other symptoms and signs of hypercortisolemia include the following: papular acne, vellus hypertrichosis of the forehead and upper cheeks, decreased libido, impotence, oligomenorrhea and amenorrhea, infertility, cutaneous and systemic fungal infections, poor wound healing, nephrolithiasis, polyuria, headaches, neuropsychiatric problems ranging from major affective disorders to global psychological dysfunction, and, rarely, spinal epidural lipomatosis.[9,23,27] Photographs antedating the appearance of signs and symptoms may support the diagnosis.[28] Features typical of Cushing syndrome may be absent in patients with ectopic ACTH or CRH secretion.[27] Ectopic ACTH secretion, however, typically presents in a boy with hypokalemia and rapid onset of symptoms.[9] Cyclic or periodic Cushing syndrome, in which the exacerbation of mild cushingoid features may parallel fluctuating hormonogenesis, is due to Cushing disease in 50% of cases.[35]
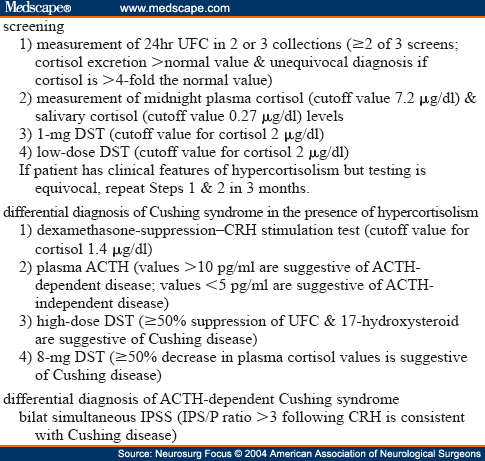
Excessive glucocorticoid levels in urine or blood are factors leading to a diagnosis of Cushing syndrome. No biochemical diagnostic test is perfectly accurate, however, and thus the clinician often must resort to different tests to distinguish Cushing syndrome from pseudo-Cushing syndrome (Table 2). Conditions that alter absorption or metabolism of the synthetic glucocorticoid dexamethasone will confound the results of the overnight DST, the low-dose DST, and the high-dose DST. Dexamethasone levels should be measured in conjunction with the morning cortisol level following administration of dexamethasone. Twenty-Four Hour UFC Level
Determination of a 24-hour UFC level by performing HPLC or an RIA is the best screening test for Cushing syndrome.[28] Two to three 24-hour collections of urine ensure the increased accuracy of the test. The diagnosis is unequivocal in a patient with classic features of the syndrome and four times the normal level of cortisol excretion (~ 400 µg/day according to most RIAs).[27] In adults, normal values are less than 80 to 120 µg (220–330 nmol) when assessed using an RIA and at least 50 µg when performing HPLC.[36] In children, the UFC level should be corrected for the patient's body-surface area. Normal values are less than 70 µg/m2 per day (37.5 ± 15.1 µg/m2/day for boys and 31.9 ± 17.6 µg/m2/day for girls).[12] In assessing women who are pregnant, one should accept a higher upper limit of normal free cortisol. Increased fluid intake will augment the amount of UFC excretion and renal insufficiency or a low urine volume will lower the amount of cortisol that is excreted.[27] The test is not reliable when creatinine clearance is less than 20 ml/minute.[28] The diagnostic sensitivity and specificity of measurements provided by HPLC range from 95 to 100% in various series.[24] False-positive results may occur in patients with pseudo-Cushing syndrome due to endogenous depression, obsessive–compulsive disorder, anxiety, chronic alcoholism, eating disorders, poorly controlled diabetes mellitus, sleep apnea, or serious illness.[26,28] Periodic endogenous hypercortisolism may vary in cycle length from 12 hours to 85 days. If cyclic Cushing syndrome is suspected based on the clinical findings and the initial biochemical evaluation is nondiagnostic, a repeated evaluation between 3 and 6 months later is recommended.[28,39]
Cortisol secretion normally follows a circadian rhythm, peaking in the early morning and reaching its nadir sometime in the late evening to a few hours past midnight.[13] In patients with Cushing syndrome cortisol secretion fails to decrease during the normal nadir period. Measurement of the plasma level of cortisol via an indwelling venous catheter distinguishes pseudo-Cushing syndrome from Cushing syndrome with a 95% diagnostic accuracy when 7.2 µg/dl (198 nmol/L) is used as a cutoff value.[31] The overall test has a 5% false-negative rate.[28]
Measurement of late-night salivary cortisol (obtained at bedtime, 11 p.m., or midnight) is as sensitive as and more convenient than the plasma cortisol test, and obviates the stress of venipuncture.[11,33] A cutoff value of 0.27 µg/dl (7.5 nmol/L) offers a diagnostic accuracy of 93%[11] which is comparable to that of a midnight serum concentration of cortisol (95.7%) and a UFC level (95.3%).[32]
In patients with Cushing syndrome suppression of cortisol secretion fails following overnight or low-dose dexamethasone administration.[36] In patients with Cushing disease, the set point for ACTH secretion is higher than normal. Thus, low doses of dexamethasone fail to suppress ACTH secretion.[36] Dexamethasone has a halflife of approximately 5 hours in plasma and between 36 and 54 hours in tissue.[40] This test consists of administering 1 mg dexamethasone (in children 15 µg/kg body weight)[20] at 11 p.m. and measuring the serum cortisol level at 8 a.m. the next morning. Following dexamethasone administration, a normal plasma cortisol level is less than 2 µg/dl (< 50 nmol/L); concentrations higher than 10 µg/ml (> 275 nmol/L) are strongly suggestive of Cushing syndrome and values between 2 and 10 µg/dl (138–276 nmol/L) are equivocal.[36] This test has a modest diagnostic accuracy due to the occurrence of false-positive results (15–20%)[20] and a sensitivity as low as 55% in cases of mild hypercortisolism.[1]
The low-dose DST consists of oral administration of 0.5 mg dexamethasone every 6 hours for 48 hours. The plasma cortisol level is measured at baseline and 48 hours after the first dose of dexamethasone. Plasma cortisol levels lower than 2 µg/dl (50 nmol/L) reportedly have a sensitivity rate of approximately 97%.[36]
Once the diagnosis of Cushing syndrome has been established, the clinician must seek to identify the cause of excess cortisol secretion. Dynamic biochemical tests help distinguish corticotropin-secreting adenomas, which retain their responsiveness to both suppression by corticosteroids and stimulation by CRH from ectopic ACTH-secreting tumors, which usually function autonomously.
Approximately 85% of patients with Cushing disease respond to ovine CRH with an increase in plasma levels of ACTH and cortisol. Only 5% of patients with ectopic ACTH-secreting tumors respond.[20] The CRH (1 µg/kg or 100 µg) is intravenously administered in the morning; this illicits an increase in plasma ACTH or cortisol levels in patients with Cushing disease, but no response in patients with ectopic ACTH secretion.[30] A rise in plasma ACTH values greater than 35% (measured at 15 and 30 minutes postinjection) compared with baseline values yields a 100% rate of specificity and a 93% rate of sensitivity. An increase of at least 20% in the cortisol level measured 30 and 45 minutes after CRH administration yields a specificity of 88% and a sensitivity of 91% in the diagnosis of Cushing disease.[30] When the CRH stimulation test is performed in conjunction with the DST, nondiagnostic results from both tests rule out a diagnosis of Cushing disease with a diagnostic accuracy greater than 98%.[29]
The dexamethasone–CRH test distinguishes patients with pseudo-Cushing syndrome from those with Cushing syndrome. Integrating the low-dose DST with the CRH test (described below) significantly increases its diagnostic accuracy.[28] This test is performed by oral administration of 0.5 mg, dexamethasone every 6 hours, providing eight doses beginning at noon and ending at 6 a.m. Corticotropin- releasing hormone (Acthrel; Ferring Pharmaceuticals, Inc., Tarrytown, NY), 1 µg/kg body weight, is given intravenously 2 hours after the last dose, and the level of cortisol is measured just before CRH administration and 15 minutes later. A plasma level of dexamethasone should be recorded before the CRH test is given to confirm the patient's normal metabolism. A plasma cortisol level of 1.4 µg/dl (38.6 nmol/L) or greater supports the diagnosis of Cushing syndrome.[28] Furthermore, Isidori and colleagues[15] have demonstrated that more than a 30% suppression of serum cortisol during the low-dose DST and/ or more than a 20% increase in cortisol during the CRH test had significantly higher rates of sensitivity (97%) and specificity (94%) than either the high-dose DST or the CRH test alone in the differential diagnosis of ACTH-dependent Cushing syndrome. Thus, the differential diagnosis between Cushing disease and ectopic ACTH secretion can be performed with a high accuracy by combining the results of the formal 2 mg/day 48-hour low-dose DST and the CRH test for serum cortisol.[15]
The advent of a sensitive and specific two-site immunometric assay for plasma ACTH has facilitated the diagnosis of Cushing disease.[7] Adrenocorticotropic hormone has a short plasma halflife, necessitating that samples be kept in an ice water bath, centrifuged, separated into aliquots, and frozen within a few hours to avoid obtaining spuriously low results.[27] Simultaneous plasma cortisol levels should be determined.[1] Using an immunometric assay, plasma ACTH levels measuring more than 10 pg/ml (2.2 pmol/L) and ACTH levels higher than 20 pg/ml (4.5 pmol/L) are indicative of an ACTH-secreting neoplasm.[27] Patients with ectopic ACTH syndrome generally have very high plasma ACTH values, although these values may overlap with those seen in patients with Cushing disease.[10] In patients with Cushing disease, 50% have a 9 a.m. plasma ACTH level within the normal reference range of 9 to 54 pg/ml (2–12 pmol/L) and the remaining patients have a slightly elevated ACTH level.[36] Due to the loss of circadian rhythm, however, nighttime ACTH secretion is abnormal. A midnight plasma ACTH level greater than 23 pg/dl (5 pmol/L) confirms the presence of an ACTH excess.[37] Plasma ACTH levels are suppressed when the source of the hypercortisolism is an adrenal cortisol-secreting tumor or a micronodular or macronodular adrenal disease.[20] Subnormal daytime plasma ACTH levels that are lower than 5 pg/ml (1.1 pmol/L) are usually present in patients with ACTH-independent Cushing syndrome.[10]
When plasma ACTH levels are higher than 10 pg/ml, the source of ACTH secretion—pituitary or ectopic—must be localized. Secretion of ACTH by corticotropinomas is usually inhibited by high-dose glucocorticoid therapy. The high-dose DST is performed by collecting a 24-hour baseline urine sample of free cortisol and 17-hydroxysteroid, administering 2 mg of dexamethasone orally every 6 hours for 2 days (in children 80–120 µg/kg/day divided into four doses every 6 hours or a maximum of 2 mg every 6 hours for 2 days),[20,36] and repeating the 24-hour urine collection during the last 24 hours of the test. The criterion of 69% suppression from the baseline value of 24-hour UFC is required to yield a specificity of 100% in the diagnosis of Cushing disease.[23,27] Urinary levels of 17-hydroxysteroid are similarly suppressed in 85% of patients with Cushing disease.[20] Paradoxical responses to dexamethasone indicate the presence of either micronodular adrenal disease or ACTH-independent Cushing syndrome.[21]
The 8-mg overnight DST is widely used because of its convenience. It consists of measuring a baseline plasma level of cortisol followed by oral administration of 8 mg dexamethasone at 11 p.m. A second specimen of plasma is obtained 9 hours later, at 8 a.m., and the cortisol level is measured. A decrease in the plasma level of cortisol that is 50% or greater—the criterion for Cushing disease—yields a diagnostic accuracy comparable to that provided by the high-dose DST.[23,27]
Inferior petrosal sinus sampling for ACTH has emerged as the most accurate and reliable means of distinguishing pituitary from nonpituitary ACTH-dependent Cushing syndrome.[4,8,10,14,17,19] The IPSS should be reserved for patients with classic clinical and biochemical Cushing disease in whom magnetic resonance imaging findings are nondiagnostic or equivocal, for patients with equivocal results from suppression and stimulation tests,[39] and for patients whose clinical presentation is consistent with ectopic ACTH secretion.[9] In experienced hands, the diagnostic accuracy of IPSS approaches 80 to 100%.[17] The procedure must be performed when cortisol levels in the peripheral circulation are elevated to suppress the normal corticotroph population of the anterior pituitary. The midnight plasma cortisol level or the amount of UFC excretion should thus be measured immediately before IPSS.[27] Peripheral CRH levels should be measured routinely to exclude the possibility of a nonpituitary CRH-secreting neoplasm as the source of hypercortisolism.[9] Concentrations of ACTH are greater in inferior petrosal sinus samples obtained from patients with Cushing disease and increase after CRH administration. Corticotropin-reducing hormone significantly reduces the number of false-negative basal results.[17] An IPS/P greater than 3 following administration of CRH is considered consistent with Cushing disease. When ACTH secretion is ectopic, values of this hormone in inferior petrosal sinus and peripheral specimens are similar and do not increase after CRH is given.[27] Most patients with ectopic ACTH syndrome have an IPS/P less than 2 and, rarely, certain patients have ratios between 2 and 3. Bilateral simultaneous sampling is essential because the maximal basal nondominant IPS/P is less than 2 in more than 50% of patients with Cushing disease and remains less than 2 after administration of ovine CRH in 33% of cases.[8] Lateralization of the pituitary microadenoma is defined by an ACTH IPS gradient greater than 1.4, before and after CRH stimulation, with positive predictive values of 74 and of 83%, respectively.[10,17] Midline adenomas may cause misleading lateralization gradients.[17] The rate of correlation of the ACTH IPS gradient with operative outcome ranges from 47 to 75%.[4,17] The IPSS has been associated with morbid and even fatal complications, including deep vein thrombosis, pulmonary emboli, and brainstem vascular damage.[3,4,17]
Sampling of the cavernous sinus has yielded a 20% false-negative rate[5] and has a higher incidence of occlusive events.[27] Jugular venous sampling is easier to perform and has a sensitivity of 88% and a specificity of 100% when the interpretation criteria is the same as those for IPSS. This approach may be used as an initial procedure with a referral for IPSS when results are nondiagnostic.[6]
Table 1. Clinical Signs and Symptoms of Cushing Syndrome
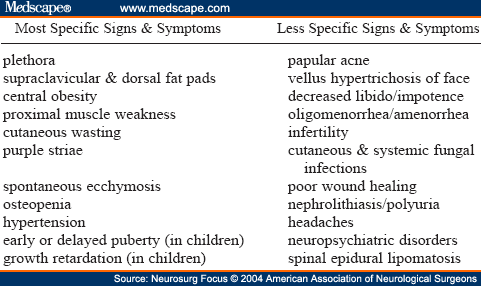
Table 2. Biochemical Diagnostic Tests for Cushing Syndrome
Reprint Address
Address reprint requests to: Marie Simard, M.D., Utah Diabetes Center, 615 Arapeen Drive, Suite 100, Salt Lake City, Utah 84103. email: marie.simard@hsc.utah.edu. Abbreviation Notes
ACTH = adrenocorticotropic hormone; CRH = corticotropin-releasing hormone; DST = dexamethasone suppression test; HPLC = high-pressure liquid chromatography; IPS/P = inferior petrosal sinus-to-peripheral ratio; IPSS = IPS sampling; RIA = radioimmunoassay; UFC = urine free cortisol
Marie Simard, M.D., Division of Pediatric Endocrinology, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City, Utah